Irritation and corrosion to the skin, loss of sight, endocrine
disruption, inflammation of airways, brain damage, burns and LD50 (lethal dose killing 50% of animals treated)… these are only a few of the repercussions of animal experimentation purely to find that perfect shade of red...
disruption, inflammation of airways, brain damage, burns and LD50 (lethal dose killing 50% of animals treated)… these are only a few of the repercussions of animal experimentation purely to find that perfect shade of red...
From tyrosine kinase inhibitors
such as imatinib (Glivec) for the treatment of chronic myeloid leukaemia, to
therapeutic antibodies such as trastuzumab (Herceptin) for the treatment of
breast cancer, to cosmetic ingredients such as Botox, animal testing has contributed
significantly to the testing of efficacy, toxicity and hypoallergenic
properties of medicinal and cosmetic products for human use.
Despite
animal rights organisations such as the RSPCA and PETA protesting against cruelty plaguing animal experimentation, many organisations/institutions believe animal use in research is fundamental to
human benefit. These include the British Heart Foundation, Cancer Research UK,
and the Royal Society. A statement published by the Royal Society (2002) (click here) proclaimed the
following:
"Modern biology, with all its contributions to the well-being of society, is heavily dependent on research on animals. Along with the great majority of the scientific community, the Royal Society considers that the benefits provide the justification for the research that led to them. At the same time, the Society also recognises that special ethical considerations are involved and that animal research must be undertaken only with the greatest care." (1)
To ensure utmost care, regulatory measures on animal testing
are being taken into high regard for animal welfare; companies/organisations in the UK have adopted the 3 R's as part of their recognition of the
“special ethical considerations” involved in exploiting animals for research (2). These don't eradicate animal experimentation, but offer a system to
ensure the soundest utilisation of animals and reduce animal suffering:
Refine. Reduce. Replace
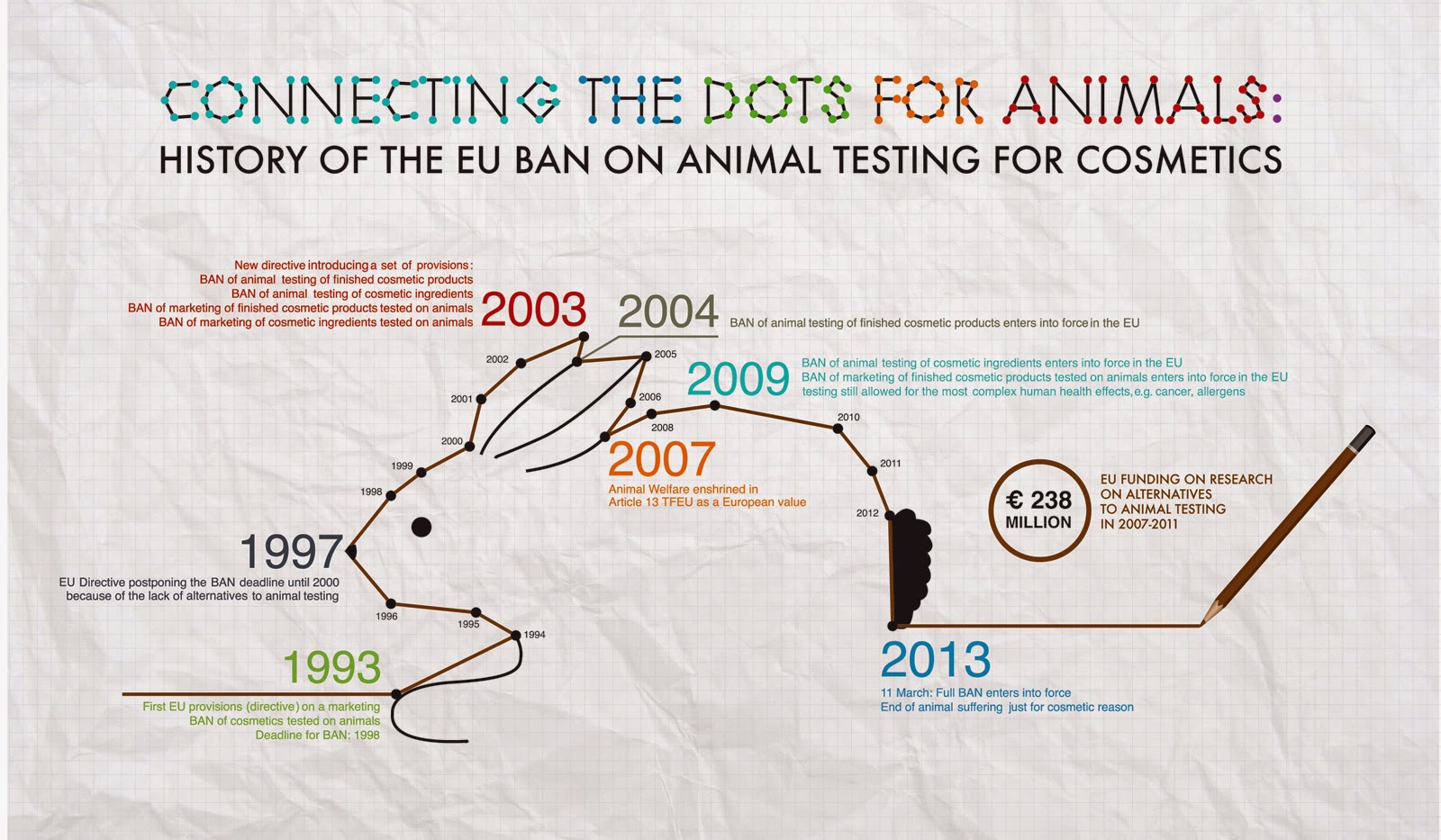
However, international companies dodge these bans;
they may still experiment on animals in other countries to achieve the
regulatory necessities of that country and continue to sell/advertise them in
other markets outside the EU. The USA and China have yet to enforce bans;
their governments require animal trials for all novel cosmetic products. The EU may not push
global brands to extract themselves from markets where animal testing still
exists, however, there has been a giant bunny leap forward for animal rights since 2013.
Alternative methods
A whopping €238 million has been spent on alternative methods
to animal testing:
- in vitro (‘test tube’) testing‒ Yet animal experiments are challenging to impersonate in vitro as they are a golden model for toxicity testing.
- Omics technologies‒ Molecular methods for detection of tissue-specific alterations when tissues are sensitive to an ingredient. They allow simultaneous examination of numerous genes, proteins or metabolites, therefore, the discovery of cosmetic-related alterations at transcriptional and translational expression levels, as seen in animal trials, is possible. Omics technologies can include: metabolomics, toxicogenomics and toxicoproteomics (5).
- Structure-activity relationship modelling‒ Predicting an ingredient’s biological properties based on its molecular structure, compared to similar ingredients that have comparable structures and modes of activity.
- Kinetic modelling‒ Mathematical method for estimating how ingredients will be absorbed, transported, metabolised, and excreted from the body.
- High-throughput platforms‒ High-speed robotic automation of human cell-based studies.
- Computational systems biology modelling‒ Human body computer simulation to explain and estimate interactions among organ systems, organs, tissues and cells.
- Cell-based assays‒ Simulate ‘circuits’ in human toxicity pathways and test for ingredient induced stresses on cells.
- Biomonitoring‒ Measuring toxic ingredients in human blood, urine or other tissues that can be used for the recognition of biomarkers that identify exposure and toxicity to an ingredient.
- Biomarkers‒ Predictive markers of biological change that can be distinguished before a toxic effect is seen in humans.
- Adverse outcome pathway (AOP) elucidation‒ Studying ingredients’ mode(s) of toxic action to detect cell targets which, when disturbed by compounds, cause toxicity. These targets can then be demonstrated in human cell studies. (6)
So can we justify the exploitation of
animals with these emerging technologies? In the end it all boils down to
one’s ethical values. In favour of animal testing? This view might bloom from a
self-examination of one’s individual and scientific intentions, and feeling
comfortable carrying out research through animal suffering. I personally believe testing of cosmetic
ingredients on animals should be banned worldwide with the emergence of alternative
testing methods. However, with regards to testing drugs such as those for
cardiovascular disease, AIDs and cancer, animals should be used in research if
human benefits are obtained that cannot be acquired with other methods, as long
as research complies with the
provisions of the Animals (Scientific Procedures) Act 1986 (ASPA).
More Information:
An in-depth debate on the pros and cons of animal testing: ProCon.org.
How animal testing has contributed to medical
science: Contribution to Medical Science
Image credit: bostonpeter7, RSPCA, European Commission
Bibliography:
(1) The Royal Society Animals in Research Committee, “The use of non-human animals in research: a guide for scientists” (2004) The Royal
Society.
(2) National Centre for the Replacement, Refinement and Reduction of Animals in Research, "Responsibility in the use of animals in bioscience research: Expectations of the major research council and charitable funding bodies"(2014).
(3) BBSRC Responsibility in the use of animals in bioscience
research report: http://www.bbsrc.ac.uk/web/FILES/Publications/animals_in_bioscience_research.pdf
(4) The European Commission ban on animal testing
page: http://ec.europa.eu/consumers/archive/sectors/cosmetics/animal-testing/index_en.htm
(5) Kroeger,
M., (2006) “How omics technologies can contribute to the ‘3R’ principles byintroducing new strategies in animal testing” Trends in Biotechnology 24(8): 343-346.
(6) The AXLR8 Alternative
Testing Strategies Progress Report 2012: http://axlr8.eu/assets/axlr8-progress-report-2012.pdf
















0 comments :
Post a Comment